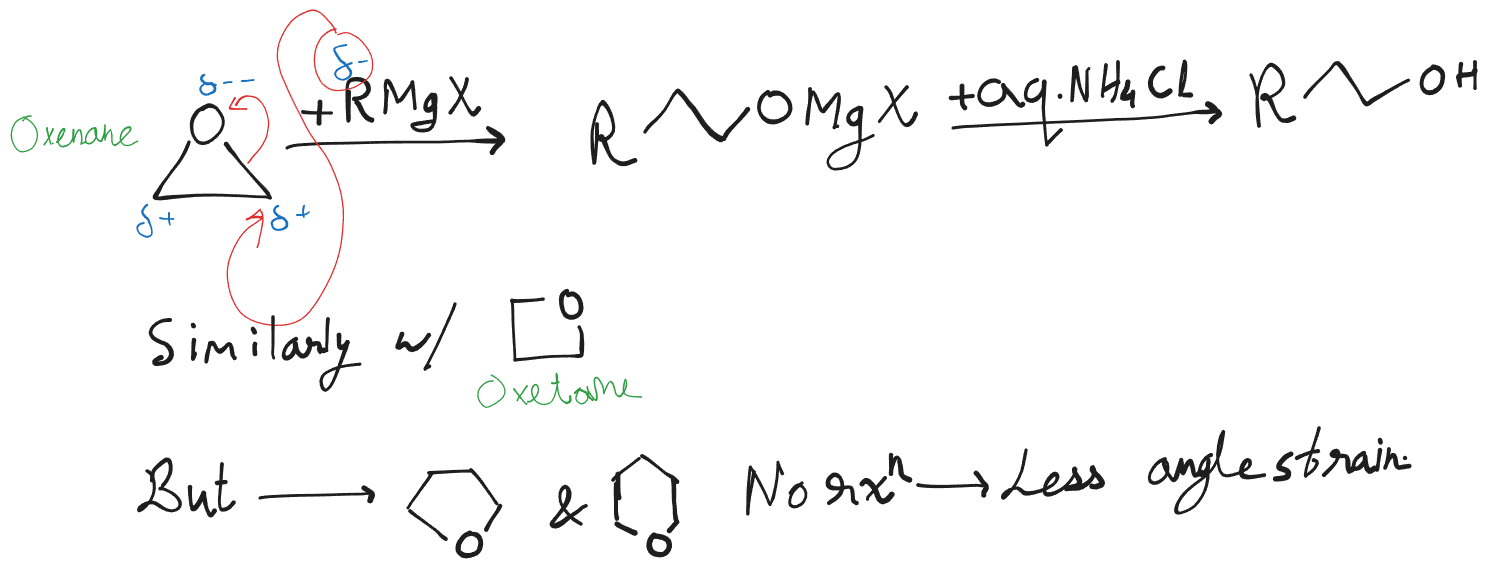From Alkenes
Acid-Catalyzed Hydration
| Atom | Attached to | Origin |
|---|---|---|
| H | C | Solvent/Acid |
| O | C | Solvent |
| H | O | Solvent |
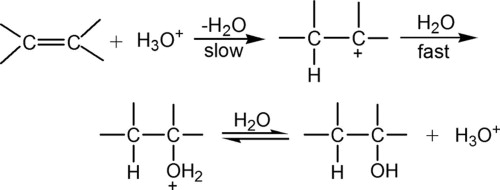
- These reactions follow Markovnikov’s Rule. And thus, are unable to produce primary alcohols except in the special case of hydration of ethene, and are reversible.
- In the case of ethene a primary alcohol (Ethanol) is formed, when ethene is treated with at 300C, and high pressure.
- It is the reverse case of dehydration of an alcohol.
- The rate determining step is the formation of carbocation.
- Rearrangement is possible due to carbocation formation.
- Not generally used to synthesize alcohols due to rearrangements causing isomeric alcohol products to form.
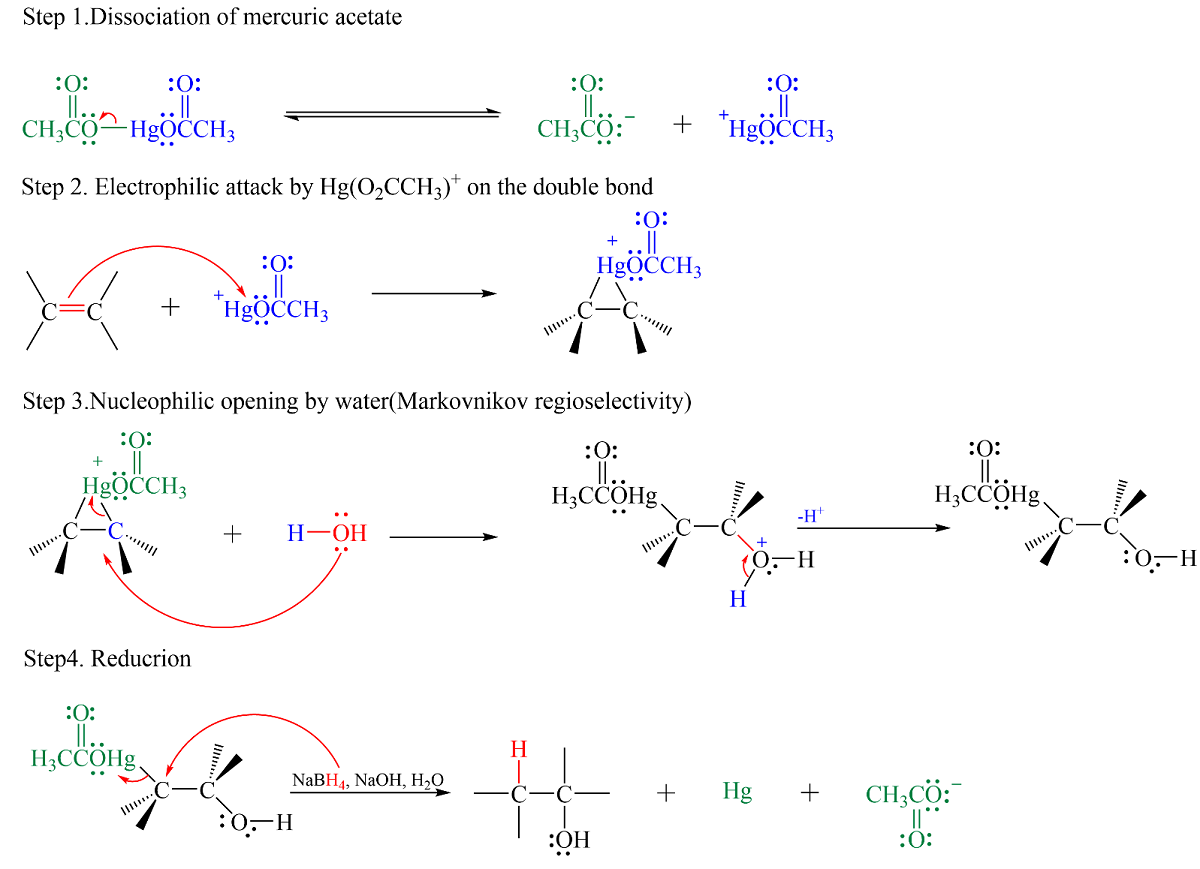
Oxymercuration-Demercuration
| Atom | Attached to | Origin |
|---|---|---|
| H | C | Hydride () |
| H | O | Added Water |
| O | C | Added Water |
- Rearrangements rarely take place (No carbocation is formed.)
- As per SHAB principles,
- An electrophilic attack by the mercury specimen at the less substituted carbon atom. (It later is replaced by .)
- is added to the more congested site.
- is added to the less congested site.
- This is in accordance with Markovnikov’s Rule.
- The H- becomes attached to the carbon with greater number of Hydrogen atoms.
- The first step is anti.
- Both steps (1→3 is Oxymercuration and 4 is Demercuration) occur in the same vessel.
- Oxymercuration ~ Few seconds - Few Minutes
- Demercuration ~ Less than an hour.
- High yield of alcohol ~>= 90%.
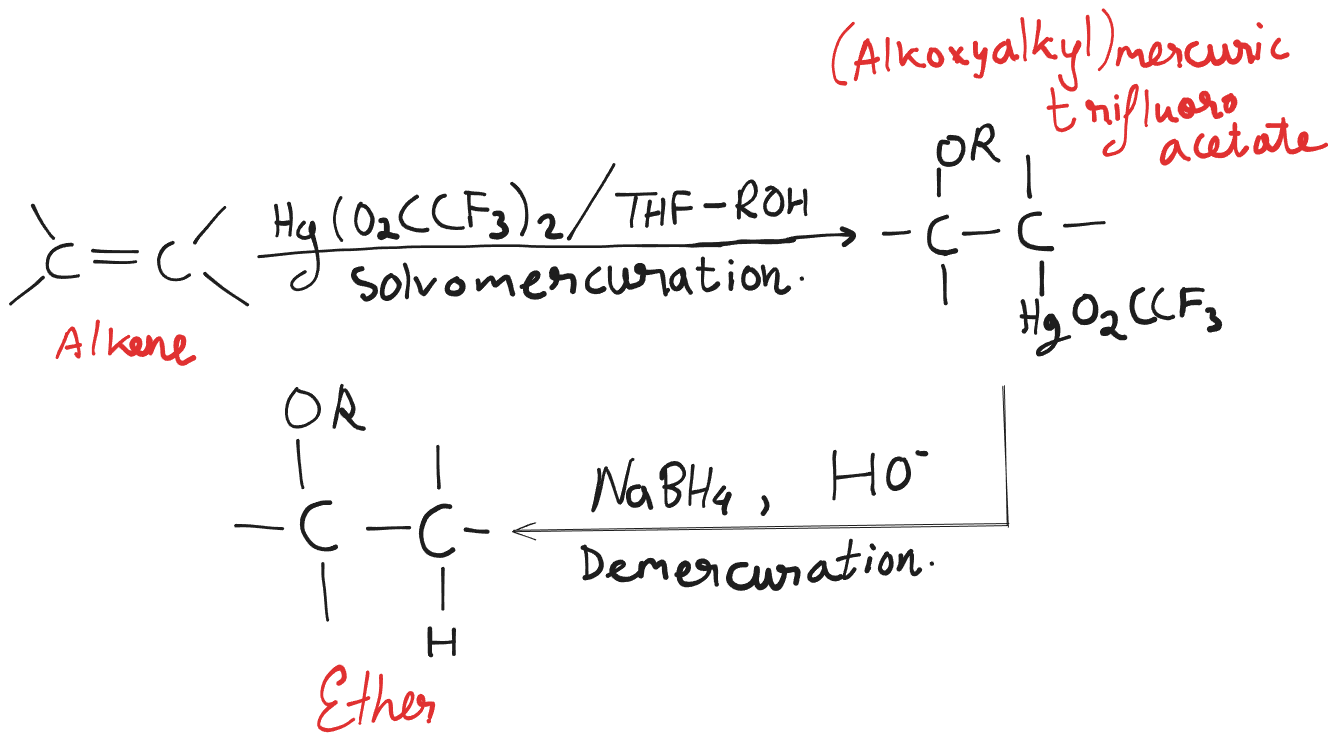
In case an alkene is treated with mercuric trifluoroacetate, , in containing an alcohol, , the product is an (alkoxyalkyl)mercury compound. Treating this product with results in the formation of an ether. This process is called solvomercuration-demercuration.
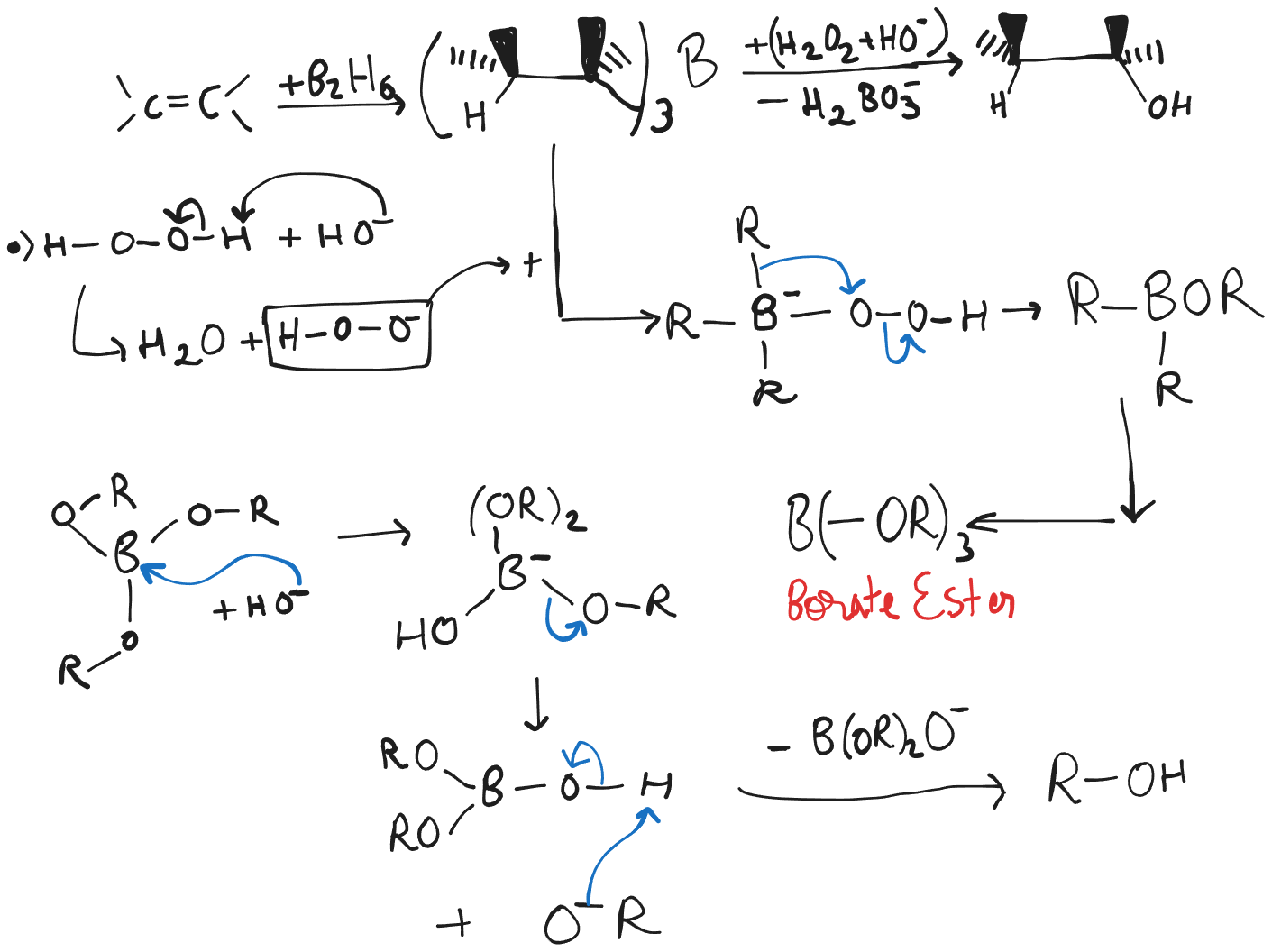
Hydroboration-Oxidation
| Atom | Attached to | Origin |
|---|---|---|
| H | O | Solvent |
| H | C | Diborane |
| O | C | Peroxide |
- Hydroboration-Oxidation can be achieved through the use of diborane () or () a solution of borane in .
- The first step is Hydroboration and the second Oxidation.
- Anti-Markovnikov Regioselectivity is followed,
- is attached to the less congested site. (This was where was attached to from initially before oxidation.)
- is attached to the more congested site. (Carbon with lesser number of hydrogens.)
- Hydroboration reactions are carried out in ethers: either in diethyl ether () or in some higher molecular weight ether such as “diglyme” (, diethylene glycol dimethyl ether.)
- Diborane and alkyl-boranes ignite spontaneously in air (with a green flame.) Hence the solution of must be used in inert atmosphere and with care.
- Both electronic and steric factors account for the Anti-Markovnikov orientation of addition.
- The bulky boron-containing group can approach less substituted carbon atom more easily.
- As the 4-membered transition state approaches, electrons shift in the direction of the boron atom and away from the more substituted atom, causing the more substituted carbon atom develop a partial positive charge. Since the carbon atom is more substituted, it is better able to accommodate the positive charge.
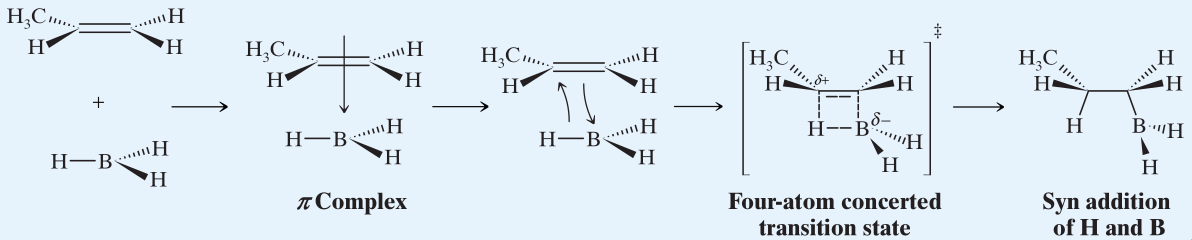
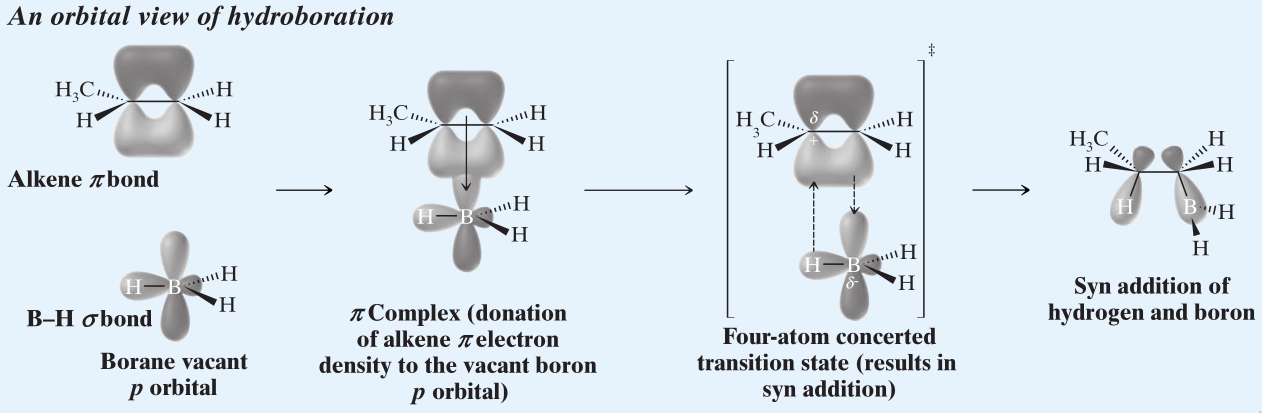
- Hydroboration requires the addition of and to the same face, causing Syn addition.
- Oxidation of the alkylborane is regioselective and is anti-markvonikov specific.
- Hydroboration-Oxidation is stereospecific and Syn.
From Carbonyl Compounds
Grignard Reagents
Grignard Reagent react with carbonyl compounds, which are highly susceptible to nucleophilic attacks, to form alcohols. Grignard Reagents are very useful since it is possible to prepare any alcohol we wish by paying special attention to the groups attached to the hydroxyl-carbon.
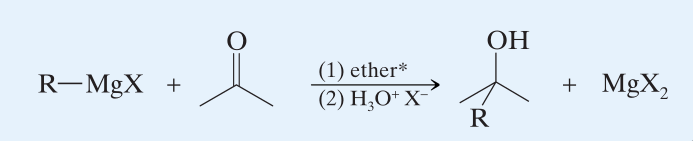
→ (Step 1) Nucleophilic addition to carbonyl group. 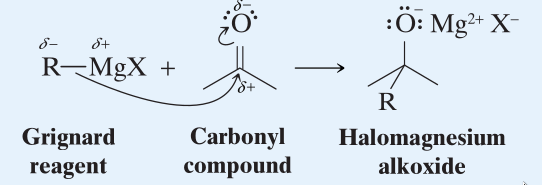 - The grignard reagent uses its electron pair to form a bond with the carbon atom.
- The previous C=O bonding pair shifts to the oxygen atom and it results in the formation of an halomagnesiumalkoxide ion.
- This reaction is done in an ether solvent.
→ (Step 2) Nucleophilic addition to carbonyl group.
- The grignard reagent uses its electron pair to form a bond with the carbon atom.
- The previous C=O bonding pair shifts to the oxygen atom and it results in the formation of an halomagnesiumalkoxide ion.
- This reaction is done in an ether solvent.
→ (Step 2) Nucleophilic addition to carbonyl group. 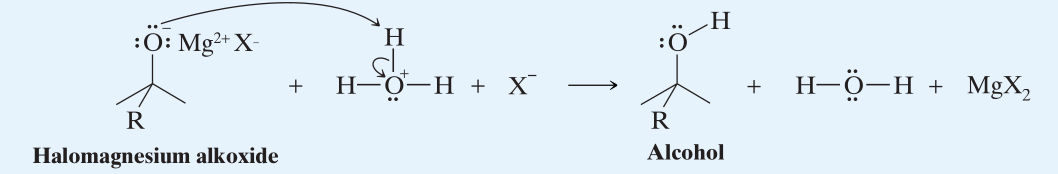 - The addition of aqueous causes protonation of the alkoxide ion.
- Note: If the alcohol is tertiary it maybe susceptible to acid-catalyzed dehydration. In such cases, a solution of in water is often used as it is acidic enough to convert to while not allowing acid-catalyzed reactions of the resulting t-alcohol.
- The addition of aqueous causes protonation of the alkoxide ion.
- Note: If the alcohol is tertiary it maybe susceptible to acid-catalyzed dehydration. In such cases, a solution of in water is often used as it is acidic enough to convert to while not allowing acid-catalyzed reactions of the resulting t-alcohol.
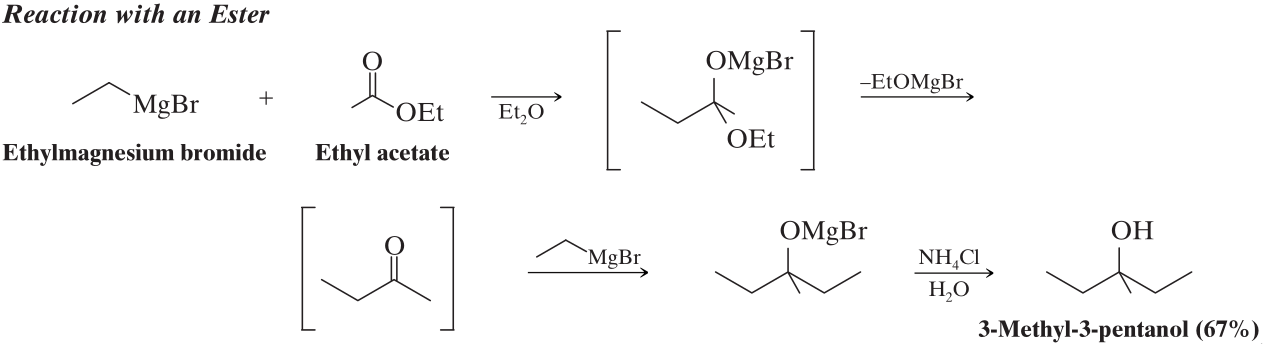
Example of a grignard reagent reacting with an ester.
Example of a few reactions:
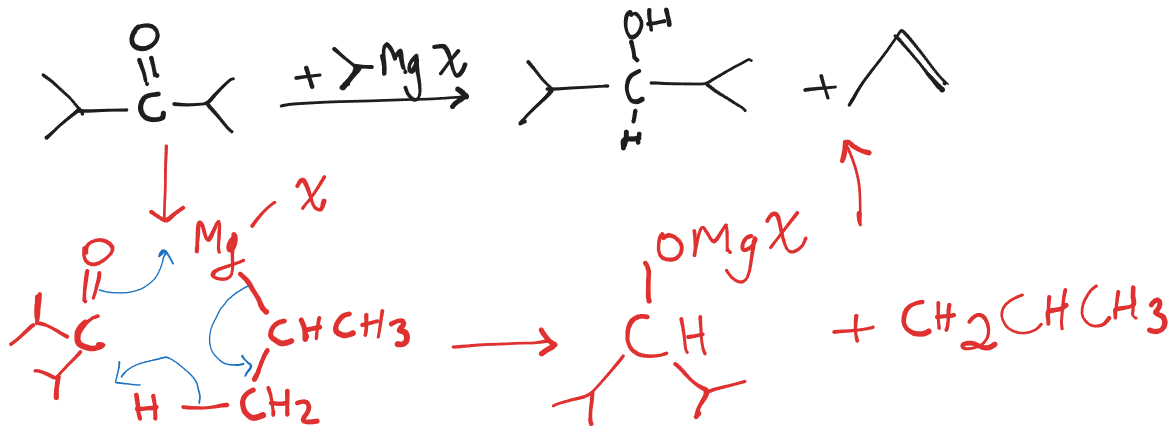
When Grignard Reagent and Substrate are both sterically hindered, the Grignard Reagent acts as a reducing agent. Presence of H, which is capable for deprotonation and the possibility of a double bond to be formed must also be there.
Addition to -unsaturated carbonyl compounds.
Presence of -branching reduces 1,2-addition heavily, and increases 1,4-addition.
Restrictions and Limitations on the use of Grignard Reagents
Grignard Reagents have extraordinary reactivity as base and nucleophile. Effectively, it contains a carbanion.
It is not possible to prepare a Grignard Reagent from a compound that contains any hydrogen more acidic than the hydrogen atoms of an alkane or alkene.
If we were to do so, the formation would either fail or instantly be neutralized by the acidic groups.
This means that when we prepare Grignard reagents we are limited to alkyl halides or to analogous organic halides containing carbon-carbon double bonds, internal triple bonds, ether linkages, and groups.
We can use the limitation of acetylenic hydrogens being acidic enough to react with grignard reagents by allowing terminal alkynes to react with alkyl grignard reagents.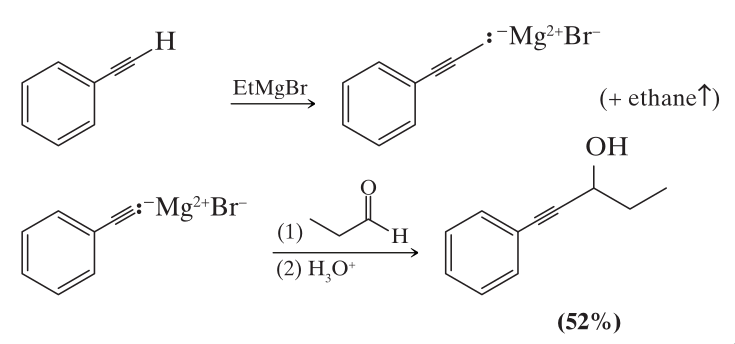
- When synthesizing, make sure to account for acid-base reactions with Grignard first.
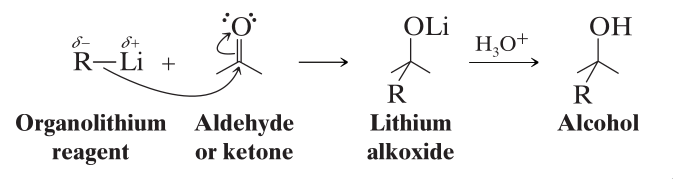
Organolithium Reagents
Organolithium Reagents have the advantage of being more reactive than Grignard Reagents, while also reacting in the same way as Grignard Reagents.
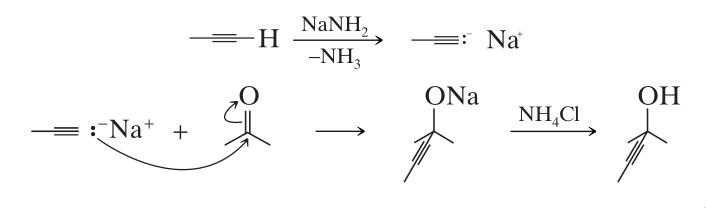
Sodium Alkynides
Sodium alkynides react with aldehydes and ketones to yield alcohols.
Lithium Aluminium Hydride (LAH) and Sodium Borohydride
Carboxylic acids and esters are especially hard to reduce, but LAH is a strong enough reducing agent to reduce carboxylic acids and esters.
LAH reacts violently with proton donors to release hydrogen gas. Hence, reductions with LAH must be carried out in anhydrous medium (such as anhyd. ether.)
- LAH reduction of an ester yields two alcohols, one from the carbonyl part, the other from the alkoxyl part.
- Sodium borohydride is usually preferred over LAH for the reduction of aldehydes and ketones, as it can react safely and effectively in water as well as alcohol solvents.
LAH
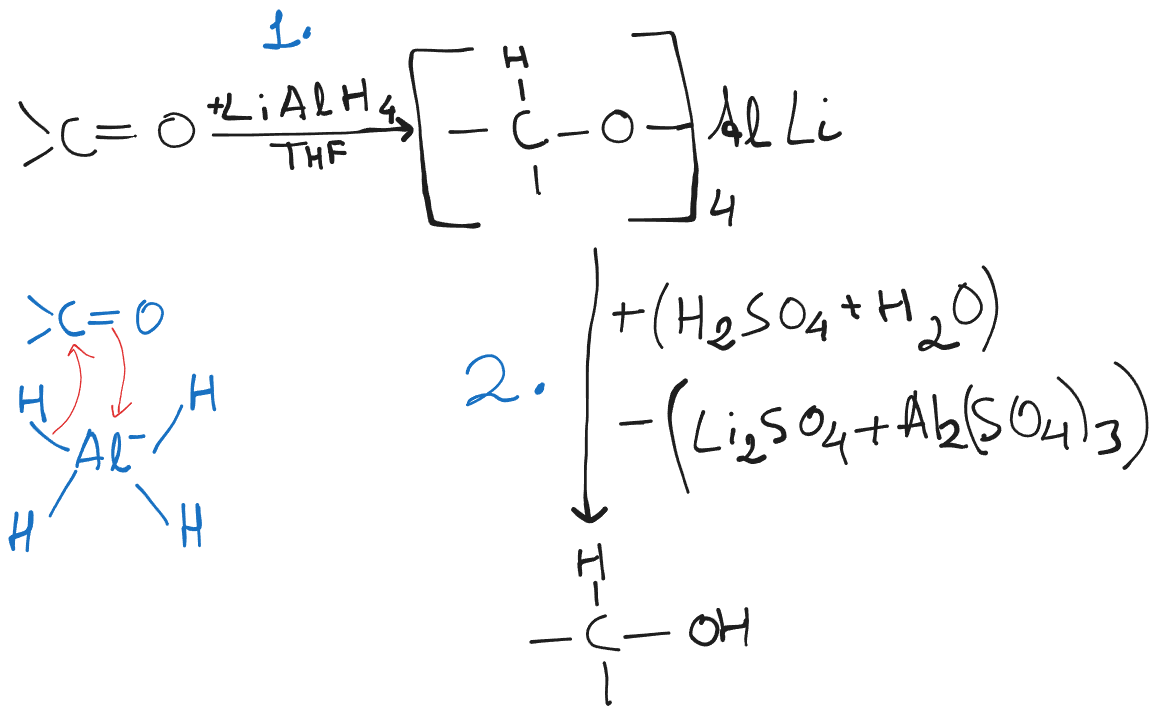
| Atom | Attached to | Origin |
|---|---|---|
| H | O | Solvent |
| H | C | Hydride |
| O | C | Initial Substrate |
- 2 reactions.
- Involves the formation of a cyclic transition state.
- In case of Carboxylic acids, both of the hydrogens are from the hydride.
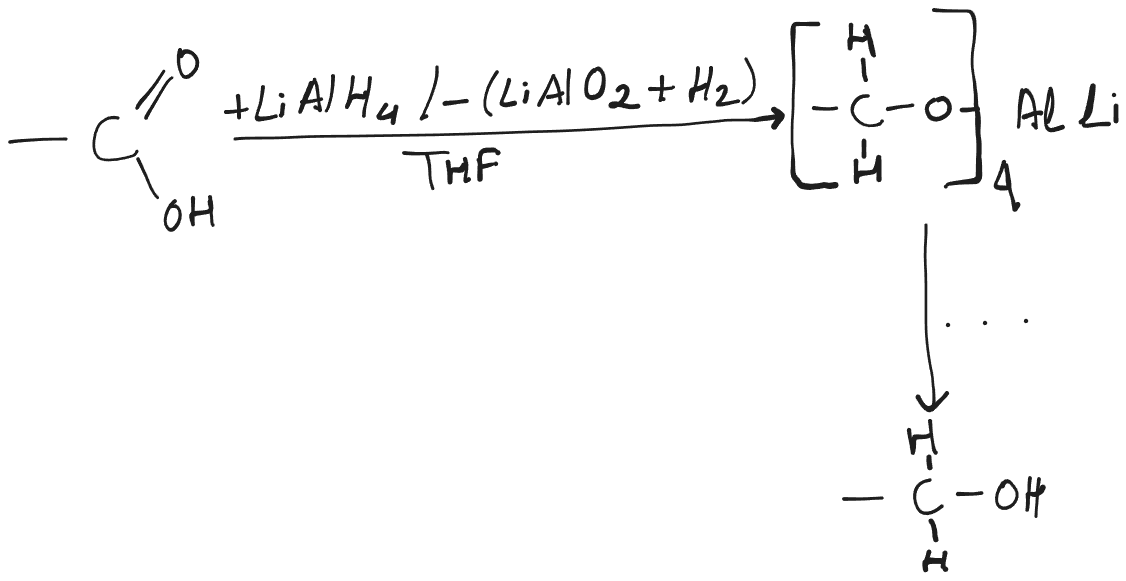
- In case of Esters, the hydroxyl group on is from the solvent and the two hydrogens and hydroxyl group on is from the hydride.

LAH can also be used to reduce alkyl halides to hydrocarbons.
Sodium Borohydride
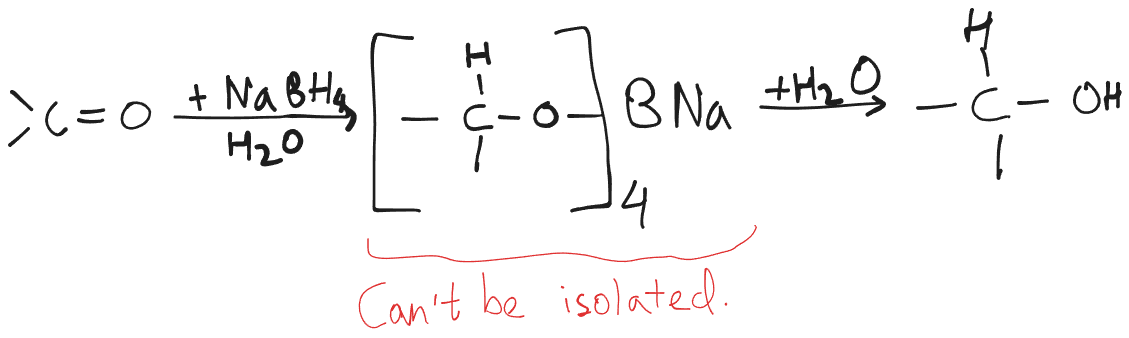
| Atom | Attached to | Origin |
|---|---|---|
| H | O | Solvent |
| H | C | Hydride |
| O | C | Initial Substrate |
- Single reaction.
- is not strong enough of a reducing agent to be able to reduce carboxylic acids and esters.
Catalytic Hydrogenation
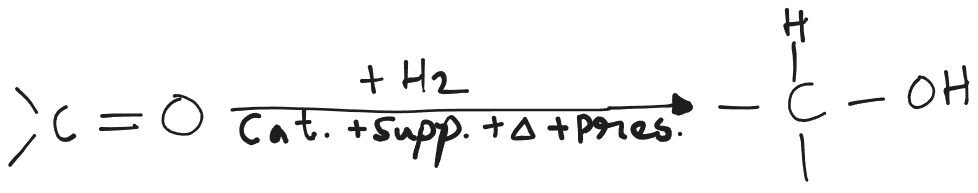
- Catalyst: , , , , .
- Support: , , .
Pinacol Coupling
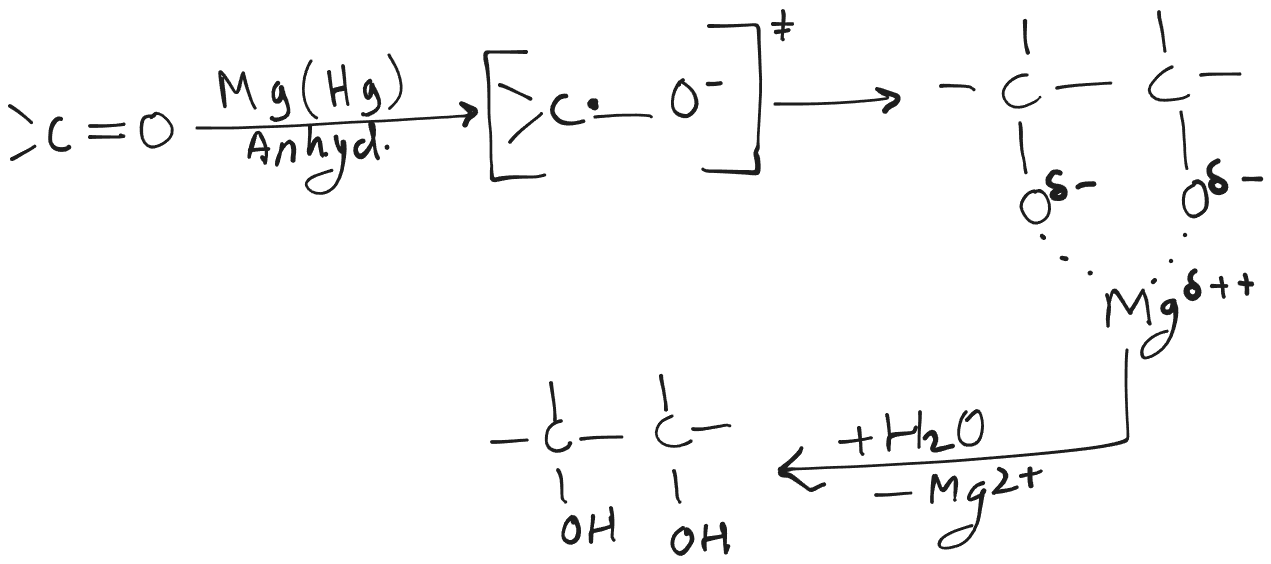
- Mg(Hg) is called magnesium amalgam.
Meerwein-Ponndorf-Verley Reduction (MPV)
It is the reverse of Oppenauer oxidation.
Bouveault-Blanc Reduction
From Amines
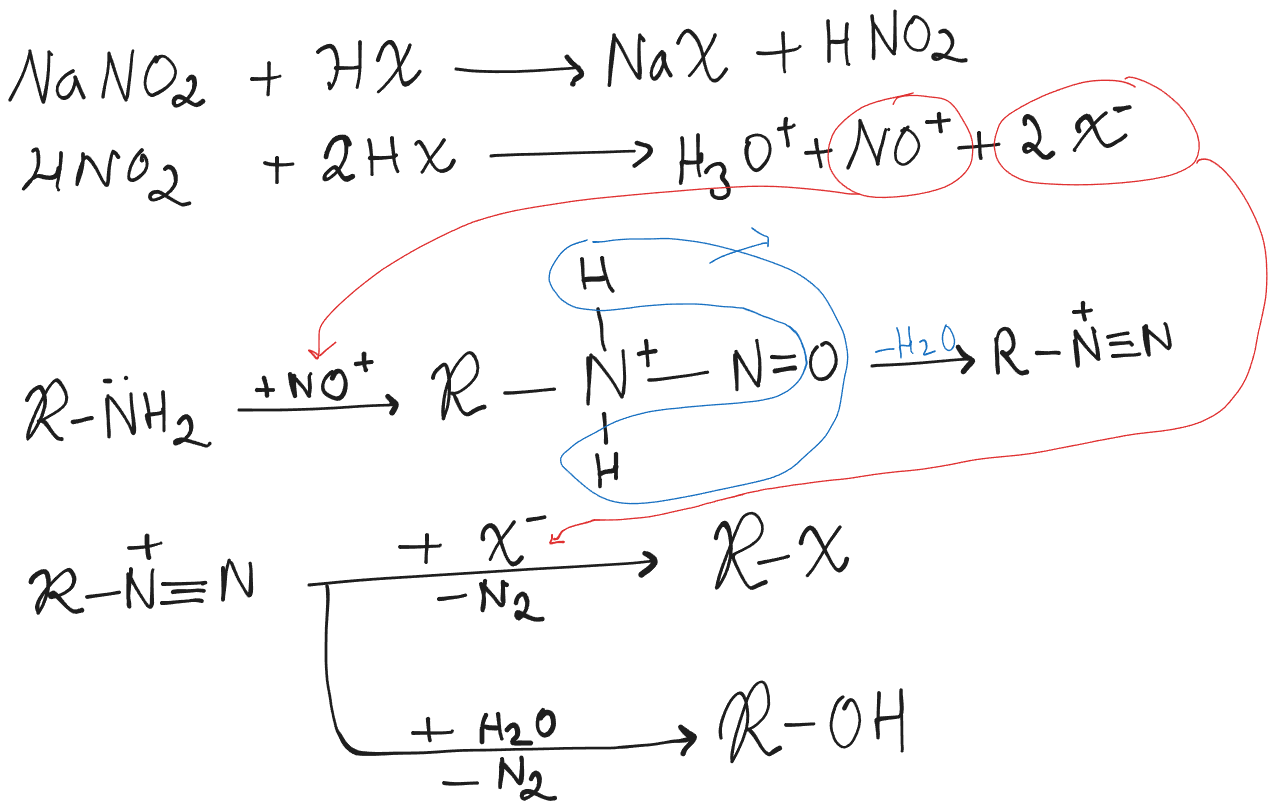 Objective is for the formation of to be less and to be more.
is a bulky species, that slows down activity. It helps reduce the formation of .
A good is . It is non oxidizing and is a bad nucleophile and therefore won’t cause substitution.
Objective is for the formation of to be less and to be more.
is a bulky species, that slows down activity. It helps reduce the formation of .
A good is . It is non oxidizing and is a bad nucleophile and therefore won’t cause substitution.