It is a versatile method for the synthesis of alkanes and other hydrocarbons from organic halides.
The mechanism is beyond the scope of our syllabus. (Take it to be )

- The alkyl halide is first treated with lithium metal in an ether solvent to convert the alkyl halide into an alkyllithium.
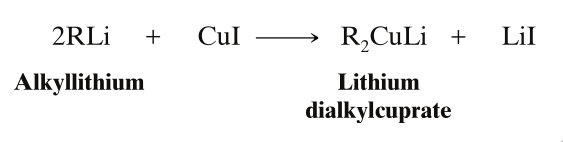
- It is then treated with cuprous iodide () to convert it into lithium dialkylcuprate.
is also known as Gilman Reagent.
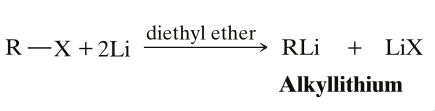
- When the Gilman Reagent is treated with the second alkyl halide, , coupling takes place between one alkyl group of the lithium dialkylcuprate and the alkyl group of the alkyl halide.
- For it to give a good yield of the alkane, the alkyl halide must be a methyl halide, a primary alkyl halide, or a secondary cycloalkyl halide.
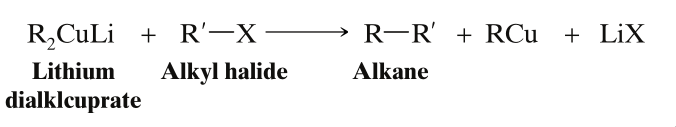
When we are preparing unsymmetrical alkanes, then the more bulky part must be taken in the form of Gilman Reagent, and the less bulky part must be taken in the form of Halide. If the opposite is done, then instead of getting the expected alkane, an alkene will be obtained due to the steric hindrance.