Most alcohols undergo dehydration to form alkenes, when heated with a strong acid. → Elimination reaction.
(Difficulty in dehydration) 1 > 2 > 3. → This is due to stabilization of carbocation. (Rearrangement is possible.)
- Secondary and Tertiary alcohols generally undergo .
- Primary alcohols generally undergo .
Dehydration of Secondary and Tertiary alcohols.
The mechanism is an reaction wherein the substrate is a protonated alcohol. → (Step 1)
- The hydroxyl group is protonated (generally happens quickly) by a hydronium ion (or any other acid catalyst.)
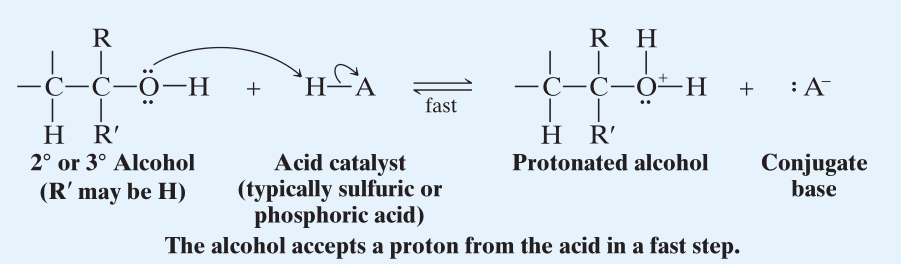
- This causes the formation of a very strong electron withdrawing group, . → (Step 2)
- The C-O bond, weakened due to the electron withdrawing nature, breaks heterolytically. This forms a carbocation and is the rate determining step.
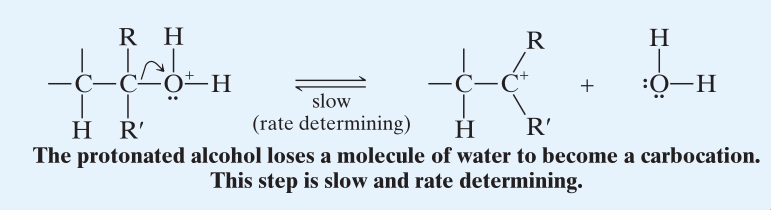
- (Formation of carbocation generally always is the rate determining step.) Hence it is this step that determines the overall reactivity of alcohols toward dehydration. Since a 1 or a 0 carbocation is not stable (hyperconjugation), their corresponding alcohols are extremely difficult to dehydrate in this manner. (Free energies of activation for the formation of carbocations from protonated alcohols: tertiary < secondary << primary.) → (Step 3)
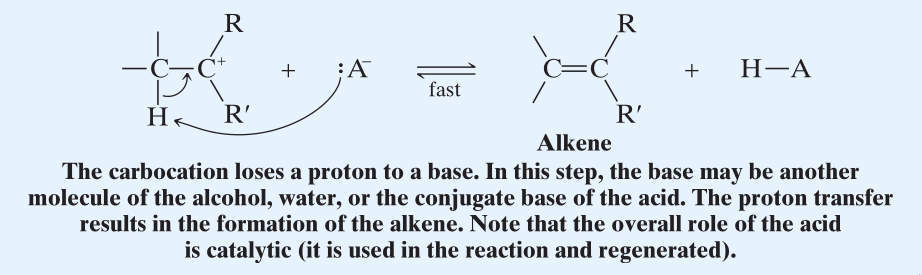
- A water molecule removes a proton from the -carbon of the carbocation. (When removing this -Hydrogen keep in mind the number of -Hydrogens of the resultant double bond.)
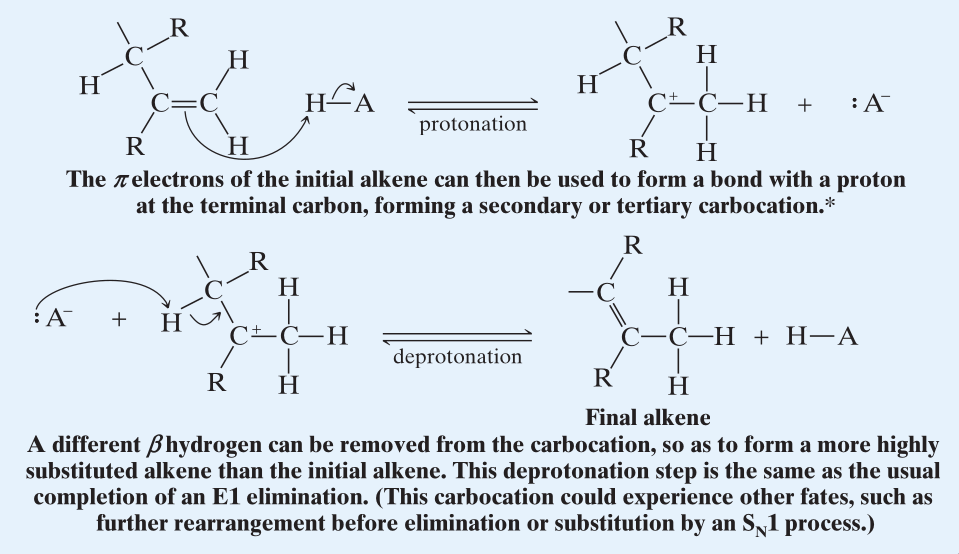
Rearrangements
- For the transition state, more stable carbocation is to be formed.
- During the removal of -Hydrogen, the more favored product is dictated by the stability of the alkene being formed. (Zaitsev’s rule.)
- Rearrangements where the carbocations have approximately equal energy are also possible.
Dehydration of Primary alcohols.
Since mechanism for primary alcohols is relatively unstable, dehydration proceeds through mechanism.
- The first step proceeds like that of and the hydroxyl group gets protonated.
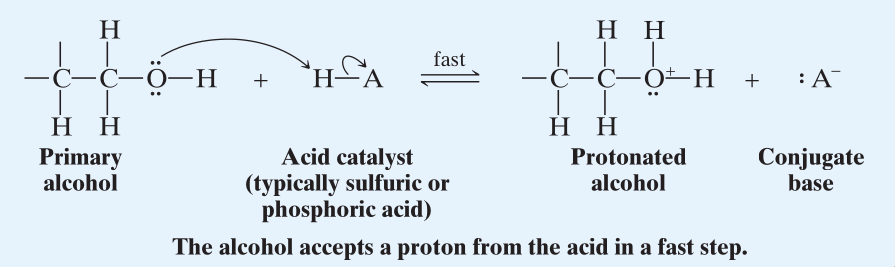
- Then with being a good leaving group, a Lewis base in the reaction mixture removes a -Hydrogen simultaneously with the formation of double bond and the departure of the protonated hydroxyl group.
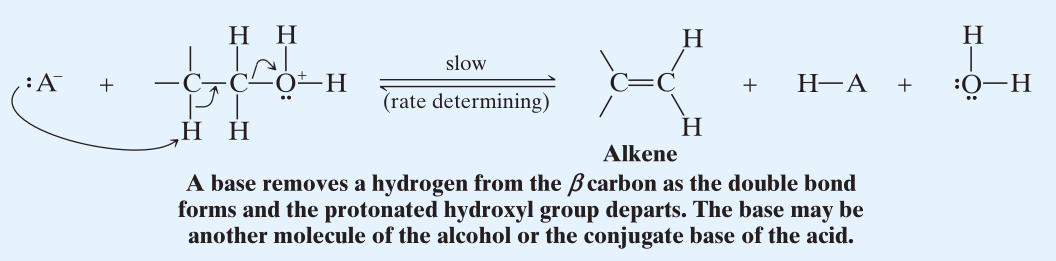
Rearrangements
- After the formation of the initial alkene the alkene can then undergo protonation, and then deprotonation again to form a different alkene.