Upon conversion to a sulfonate ester derivative, the hydroxyl group can be a good leaving group.
- Methanesulfonate Esters (mesylates)
- p-Toluenesulfonate Esters (tosylates)
- Trifluoromethanesulfonate Esters (triflates)
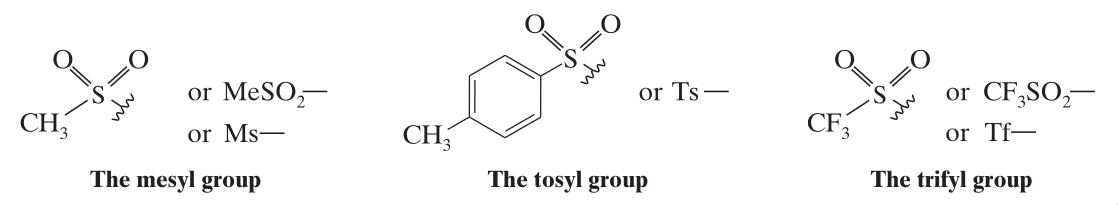
They are usually prepared by the reaction of the alcohol in pyridine with the appropriate sulfonyl chloride.
- This reaction does not affect the stereochemistry of the alcoholic carbon ⇒ retention of configuration. They are often used as substrates for nucleophilic substitution. They are good leaving groups as the sulfonate ions they become when they depart are very weak bases.
Formation:
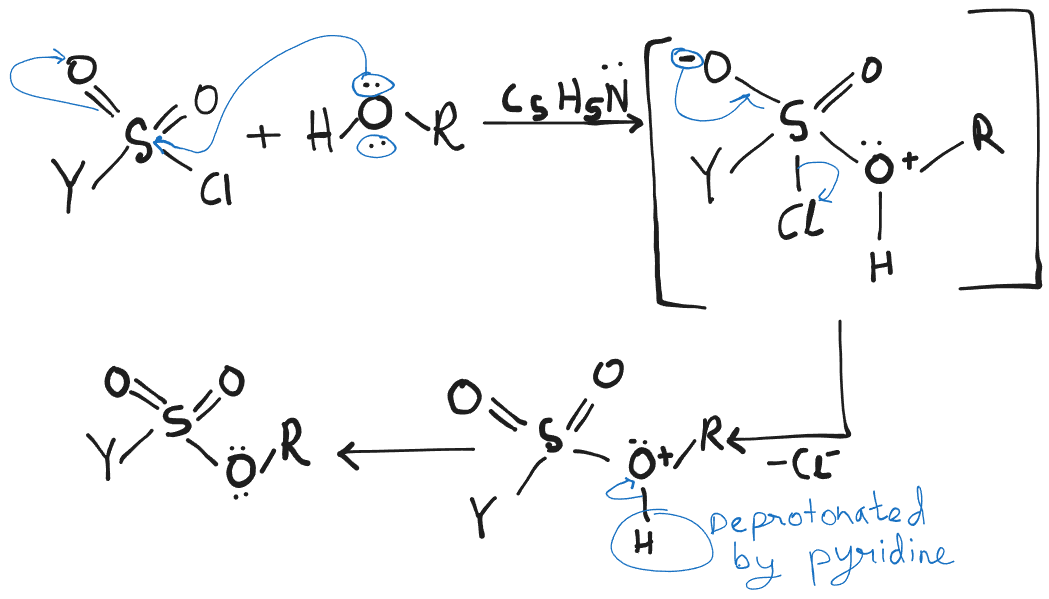 Here is Methane, p-Toluene or Trifluoromethane.
Here is Methane, p-Toluene or Trifluoromethane.